COVID-19 Immune Response Study Could Lead to More Effective Treatments
 subramanian.isbscience.org/2020/10/28/covid-19-immune-response-study-could-lead-to-more-effective-treatments/
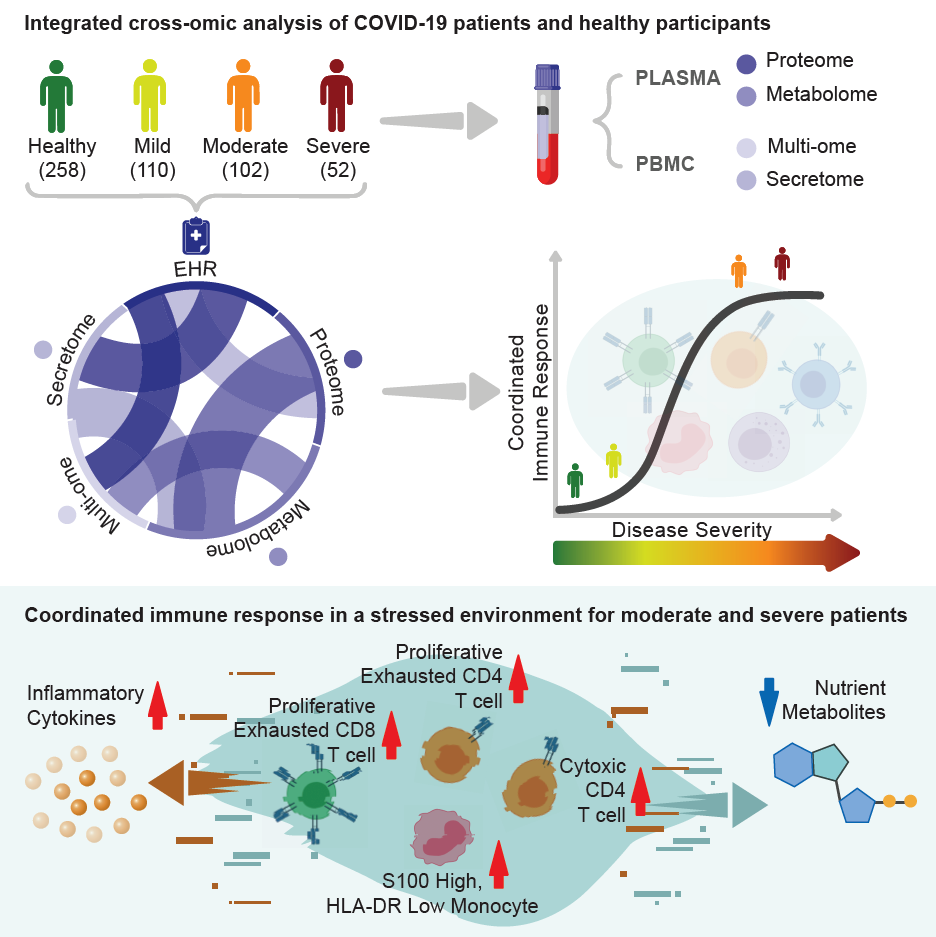
subramanian.isbscience.org/2020/10/28/covid-19-immune-response-study-could-lead-to-more-effective-treatments/The ISB-Swedish COVID-19 Immune Response Study has revealed new findings that suggest that treatments aimed at arresting the infection at the stage of moderate severity may be most effective. The symptoms of COVID-19 vary widely, from very mild to severe conditions requiring ICU care. Researchers comprehensively studied a large number of patients during the week following a COVID-19 diagnosis, and found that mild COVID-19 is very distinct from the moderate or severe forms of disease, which appear surprisingly similar.
For both moderate and severe COVID-19 cases, the team found that there is a sort of tug-of-war taking place, in which inflammation is promoting a stronger immune response, yet many of the key nutrients required for building that response are depleted. This leads to unusual and dysfunctional immune responses.
Graphical abstract published by Cell detailing findings from ISB-Swedish COVID-19 Immune Response Study
A paper describing these findings has been accepted by Cell, and were published online. The study was led by ISB and Swedish, with help from Merck (known as MSD outside the United States and Canada), BARDA, and several other institutions and companies (listed below).
“These findings have practical implications for treatment of patients with COVID-19. Since patients with moderate illness have not yet developed end organ damage, our data suggest that early in the disease course would be the best time to intervene with various treatment options to prevent the immune, protein and metabolite derangements seen with more severe disease,” said Dr. Jason Goldman of Swedish, who is the clinical lead for the study. “Our translational data align with data from randomized control trials, which have shown greater benefits from antiviral therapies given early. These data also provide intriguing hypotheses about the targeting of host-directed therapies, or even nutritional supplementation.”
The research team examined serial blood draws from 139 COVID-19 patients of all severities, from patients recovering at home to critically ill patients and in the ICU. From each blood sample, they measured thousands of proteins and metabolites to capture the environment of the circulating immune system. They also measured thousands of genes and proteins from individual immune cells. Finally, they utilized novel computational methods to merge all of these observations together to provide an integrated view of COVID-19 infection during the week following initial diagnosis.

Corresponding author and ISB President Dr. Jim Heath and lead author Dr. Yapeng Su.
The power of systems biology
“This is what we mean by ‘systems biology’ — thoroughly measure every component of the whole system, and then use computational methods to reassemble it back together again,” said ISB President Dr. Jim Heath, who was the scientific lead of the study.
“The resources provided from this work could provide high value in developing new therapies that might target metabolite starvation, immune dysfunction, or blood clotting, each of which we see emerge at the level of moderate disease,” said Dr. Yapeng Su, an ISB research scientist and lead author on the study.
“This important and comprehensive study, in which Merck has been proud to participate, demonstrates the power of an integrated systems biology approach to dissect the complexity of molecular and cellular responses in patients suffering from COVID-19,” said Dr Roger M Perlmutter, President Merck Research Laboratories. “As we had hoped, the analysis defines a possible point of intervention in the progression of COVID-19 disease, which may in the near term permit the development of more effective, targeted therapeutics.”
The COVID-19 Immune Response Study is made up by ISB, Swedish, Merck, Stanford University, Fred Hutchinson Cancer Research Center, Adaptive Biotechnologies, Bloodworks Northwest, Gilead, Isoplexis, Metabolon, Nanostring, Olink, Providence Molecular Genomics Laboratory, Scisco Genetics and 10x Genomics.
Funding for this project comes from Merck and the Biomedical Advanced Research and Development Authority (BARDA), the Wilke Family Foundation, the MJ Murdock Charitable Trust, the Swedish Medical Center Foundation, the Parker Institute for Cancer Immunotherapy, Gilead, Novartis, Amazon Web Services, Omeros, the Washington State Andy Hill CARE Fund, the Department of Defense, and the National Institutes of Health.
Note: If you are a journalist and would like to request an interview, please email ISB Communications.




